In today’s blog, our team discusses how fluorescence comprises the interrelated processes of absorption, excitation, and emission as well as how these processes work and their application.
Fluorophores
Fluorescence occurs at the molecular level when the molecules and atoms in a photoluminescent material absorb light at a particular wavelength and then emit light of a longer wavelength. Molecules that are capable of fluorescence are known as fluorophores.
Fluorophores absorb light energy at particular wavelengths and energy levels (absorption), reach an excited state (excitation), and then emit light at longer wavelengths at a lower energy level (emission)—usually in the form of visible color.
The absorbed light and emitted color of a fluorophore vary according to the fluorophore’s specific chemical structure. Fluorophores can absorb a range of wavelengths of light energy and also emit a range of wavelengths. Most colors in the visible spectrum can be produced by different fluorophores.
Absorption and Excitation
Absorption and excitation are closely interrelated. If a fluorophore does not absorb light energy, the excitation process cannot occur, and therefore fluorescent emission will not occur.
Absorption and excitation can be measured: An absorption spectrum shows all of the wavelengths at which light is absorbed by a fluorophore, while an excitation spectrum shows the wavelengths of light that a fluorophore will absorb to be able to emit at a specified wavelength. Generally, both the absorption and excitation spectra of a fluorophore will have the same peak wavelength, although there are exceptions.
When a fluorophore absorbs light in its excitation spectrum, the electrons in the fluorophore molecules move from a low energy state, known as the ground state, to a higher energy state, known as the excited state.
This excited state is unstable, so the electrons will drop back to their ground state. It is at this stage that fluorescent emission occurs.
Emission
As the electrons drop back to their ground state, they release photons that produce light at a longer wavelength than the light source. When energy between an excited state and the ground state is lost as a photon, this is known as radiative relaxation. Fluorescence is an example of radiative relaxation. In this phase, excess energy is lost as the fluorophore relaxes, the vibrational structure breaks down, and the emission spectrum appears as light, usually a color visible to the human eye. The process repeats as long as the excitation is maintained. As you’d expect, emission can be measured and represented on the emission spectra.
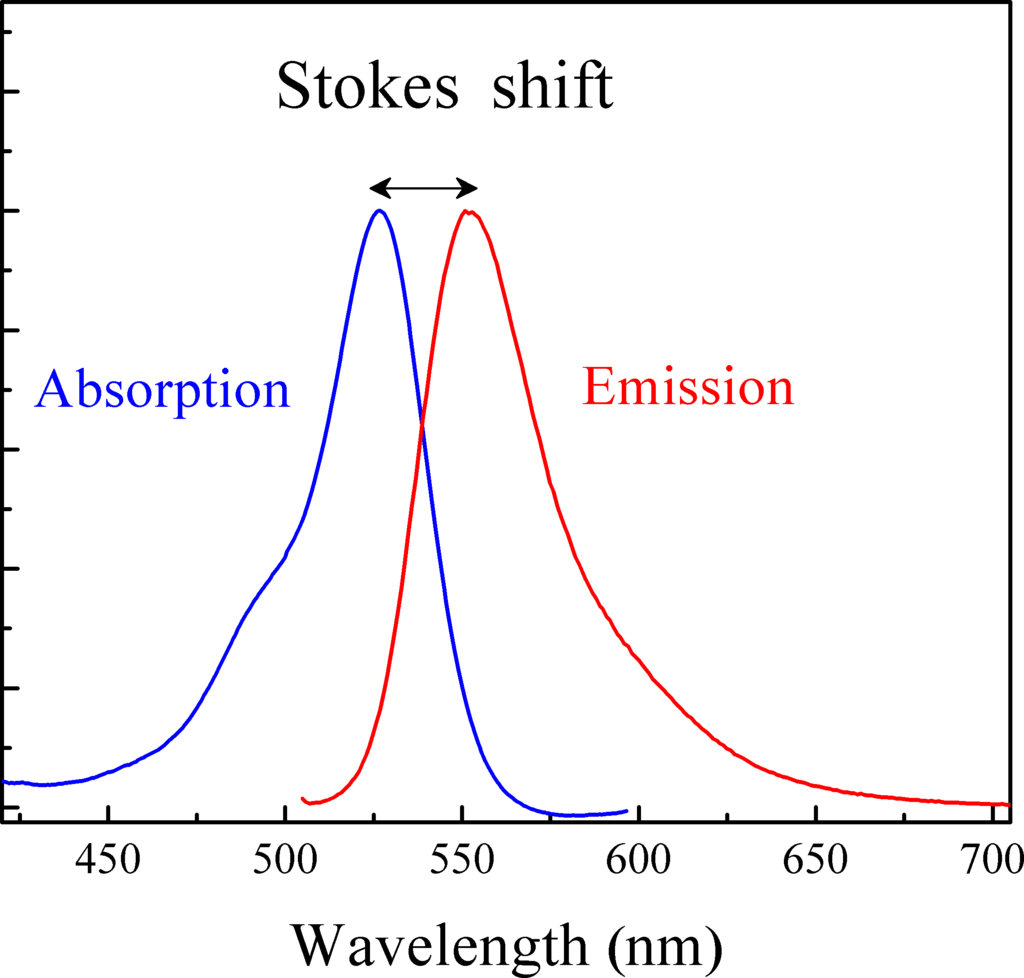
The Distance Between Excitation and Emission: Stokes Shift
Generally speaking, Stokes shift is the difference between the excitation spectrum and the emission spectrum during the same fluorescent process. In many applications, a greater or larger Stokes Shift is beneficial since it can reduce the excitation and emission processes from interfering with each other (i.e., self-quenching that results from molecular self-absorption).
The graph below shows the absorption/excitation spectra on the left in blue and the emission spectra on the right in red. Stokes shift is indicated as the distance between the peaks of the two spectra.
Application: Putting it all Together
In summary, a fluorophore absorbs light energy of short wavelength and high energy, it becomes excited and then emits light at a longer wavelength and lower energy. The time for the absorption, excitation, and emission cycle for a fluorophore is extremely fast: the entire process occurs in billionths of a second.
In many applications, Ultraviolet (UV) wavelengths that are not visible to the human eye are often used to excite fluorophores that will then emit a visible color. Fluorophores possessing these characteristics make them ideally suited for various applications such as document security, anti-counterfeiting, process control, and brand authentication.
Angstrom Technologies, Inc., designs and produces many fluorescent materials ideally suited for integration with printable inks, varnishes, adhesives, or plastics. Many finished products are designed to have little or no color under normal light and will emit an intense color only when they are exposed to invisible ultraviolet light. These materials are only detectable for as long as the excitation is maintained.
For more information on this topic and how our products can be customized for your application, contact us or give us a call today!